La plata, el principe de la joyería.
Está claro quén es el rey. Pero en segundo lugar, y durante muchos años en la historia, el primero, la plata ocupa este sitio. Más adelante hablaremos de un desconocido, el platino. Pero será otro artículo.
Vamos a hablar de:
2. Propiedades físico-químicas de la plata
1. Qué es la plata.
Es uno de los metales de la tabla periódica al que se le puede dar la denominación oficial y oficiosa de “metal precioso”. Como indicamos ya en la segunda entrega (metales preciosos II: el oro), en el artículo primero, del capítulo I de la ley 17/1985 de 1 de Julio, como en el Real Decreto 197/1988, de 22 de febrero, Título I, Capítulo 1, artículo primero, nos queda claro que para el mundo joyero y el Estado, sólo hay objetos fabricados en metales preciosos por 3 elementos, y sus aleaciones. La plata, es el tercero nombrado.
1.A. Ley de la plata
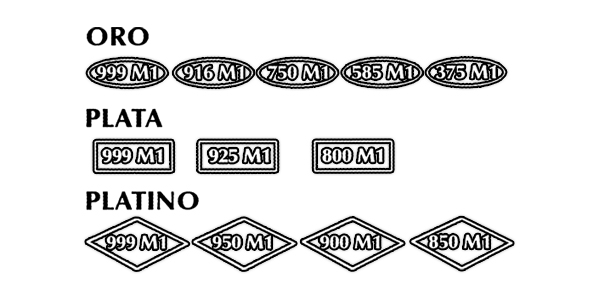
Existen dos purezas o leyes que se comercializan en España (aunque personalmente, sólo he llegado a ver una).
Si tenéis dudas sobre qué es la ley o pureza de un metal precioso, consulta este enlace.
Según esta normativa, el símbolo que representa el punzón para el contraste es un rectángulo de las siguientes características que nos indica le Real Decreto 197/1988.
«c) Para la plata será un rectángulo horizontal, cuyo lado mayor será de longitud doble que la del menor, en su interior y en el sentido del lado mayor llevará la ley del metal y a la derecha la contraseña del laboratorio que haya realizado el contraste; este punzón se construirá en dos tamaños, uno con el lado mayor de 4 milímetros y otro en que dicho lado tenga 2 milímetros.»
Todas las piezas que se comercializan como plata de 1º o 2º ley, tienen que tener como mínimo, dicha proporción de plata uniformemente repartida. Si no cumple así, no puede considerarse de dicha ley, y tendrá otra denominación que dejará clara que no tiene esa calidad.
2. Propiedades físico-químicas de la plata.
Es un elemento químico que pertenece a los metales de transición en la tabla periódica, con un número atómico 47. Su símbolo es Ag, que proviene del latín “argentum”, que significa “blanco”, “brillante.
El un elemento blando (dureza, en la escala de Mohs, 3,0), brillante (luminosidad), blanquecino (color), pesado (densidad alta, 10,490 kg/m3), maleable (permite realizar láminas muy finas) y dúctil (podemos crear hilos). Y como el oro, se presenta aislado de forma natural, pero también combinado con otros metales, en forma de sulfuro.
Como curiosidad, presenta la más alta conductividad eléctrica y térmica dentro de los metales, así como el mayor índice de reflexión. Otros datos son: peso atómico de 107, una estructura cúbica centrada en sus caras, un punto de fusión de 961 grados Celsius, y de ebullición 2162 ºC. No reacciona con la mayoría de los productos químicos, pero es sensible al ozono, sulfuro de hidrógeno o aire con azufre.

3. Aleaciones de plata.
La plata se trabaja normalmente en 925 milésimas. La aleación más común es la de plata/cobre. Uno de los problemas de las aleaciones de plata es que su «oxidación«, siendo bastante rápida. Y lo ponemos entre comillas ya que a través de este artículo hemos descubierto que el proceso de ennegrecimiento de la plata no es una oxidación, si no, sulfuración. Está extendido el termino oxidación, pero no es correcto.
3.A. Oscurecimiento de la plata.
Si leéis en la propiedades físico-quimicas anteriormente descritas, la plata reacciona al sulfuro de hidrógeno. El H2S es una sal que no contiene oxigeno y que se encuentra disuelta en algunos tipos de aguas. Así como, es un producto de degradación bacteriana de la materia orgánica en condiciones anaeróbicas (sin oxigeno).
Tras reaccionar la plata y el sulfhídrico, se forma sulfuro de plata (Ag2S) sobre la superficie y la plata empieza a adquirir un color negruzco.
Cuanto más oscura se ponga la superficie, más calor absorberá y más rápida ocurrirá la reacción de sulfuración. Cuando terminemos este monográfico, hablaremos sobre los factores que hacen oscurecer a la plata.
Actualmente existen en el mercado de la orfebrería hay una gran diversidad de objetos de plata recubiertos por una laca especial que impide su oscurecimento, manteniéndolos blancos y brillantes como el primer día.
Sin embargo, en la joyería, se obtiene resultados interesantes gracias al baño de rodio, para darle un aspecto similar al oro blanco, y que mantiene durante bastante tiempo el color.
Fuera de las aleaciones hay otros términos que utilizamos, pero que no son plata en sí; como la plata inglesa o la alpaca, que pueden confundirse con tipos de plata, pero no es así. Son conocidos dentro del menaje en plata (cubiertería, marcos, etc.), pero que hablaremos de ellos más adelante.
4. Valor de la plata.
La plata ha sido un metal que durante mucho tiempo tuvo una influencia muy fuerte en la historia y evolución de los paises. Hoy en día, su valor, está unida en gran media al precio del oro.
Las medidas en las que se comercializa la plata son las mismas que explicamos en el anterior artículo en el que hablamos del oro (onzas troy por dólares). Aquí os dejamos una evolución del valor de la plata desde el siglo XIV, y como ésta era de mayor relevancia que el oro.
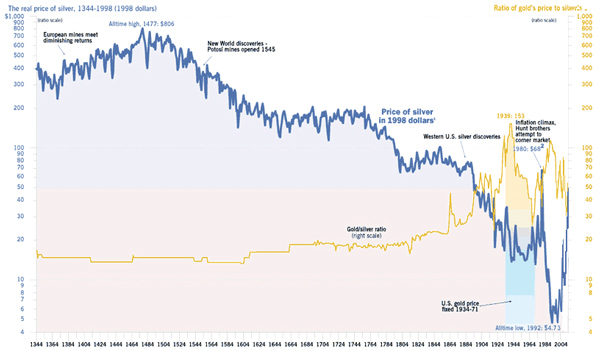
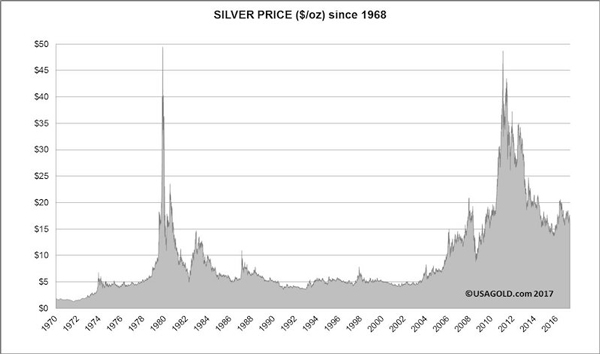
5. Historia y curiosidades.
La plata ha estado ligada de forma extraordinaria a la historia de nuestro país, España. Gran parte de ella llegaba del Nuevo Mundo (continente americano), a través de los puertos de Palos de la Frontera, Cádiz y Sevilla; y la “malgastabamos” en el Viejo. Argentina (Rio de la Plata) , las minas de Perú (Potosí) o la plata mejicana son algunas de las huellas que ha dejado este metal en el nuevo continente.

Tambíen merece un comentario sobre su uso en fotografía. Gracias al nitrato de plata, la fotografía tal y como la conocemos, existe. Dichas sales son sensibles a la luz, reaccionando al contacto con esta, permitiendo «fijar» la imagen a un soporte físico.
Otro uso extendido en nuestra vida diaria ha sido la sustitución del mercurio, muy tóxico si se expone en la naturaleza, por el oxido de plata en las pilas botón.
Algunos artículos interesantes para completar este post, nos ha resultado interesante o hemos utilizado como fuentes:
- Historia sobre las minas de Potosí.
- Cómo funcionan las pilas botón.
- Sobre la plata como mineral.
- Por qué la plata se pone negra.
- Biblioteca de joyería Raul Ibarra.
- Identificación de plata inglesa.
Nos despedimos hasta la siguiente entrega. Esperamos que os haya sido tan útil e instructivo como para nosotr@s. Siempre aprendemos algo nuevo todos los días. Hasta pronto:)